Recently, Hippocrates , a leading provider of digital solutions in the life health sector, announced the launch of its new-generation Document Management System (DMS). This innovative product is designed to provide more efficient, secure, and flexible document management solutions for large group enterprises, helping them achieve centralized and intelligent management of information resources in the process of digital transformation. The all-new DMS excels in supporting flexible group structures, robust permission management, and seamless support for Marketing Authorization Holder (MAH) collaboration.
Hippocrates's New-Generation DMS System: Distinctive Features
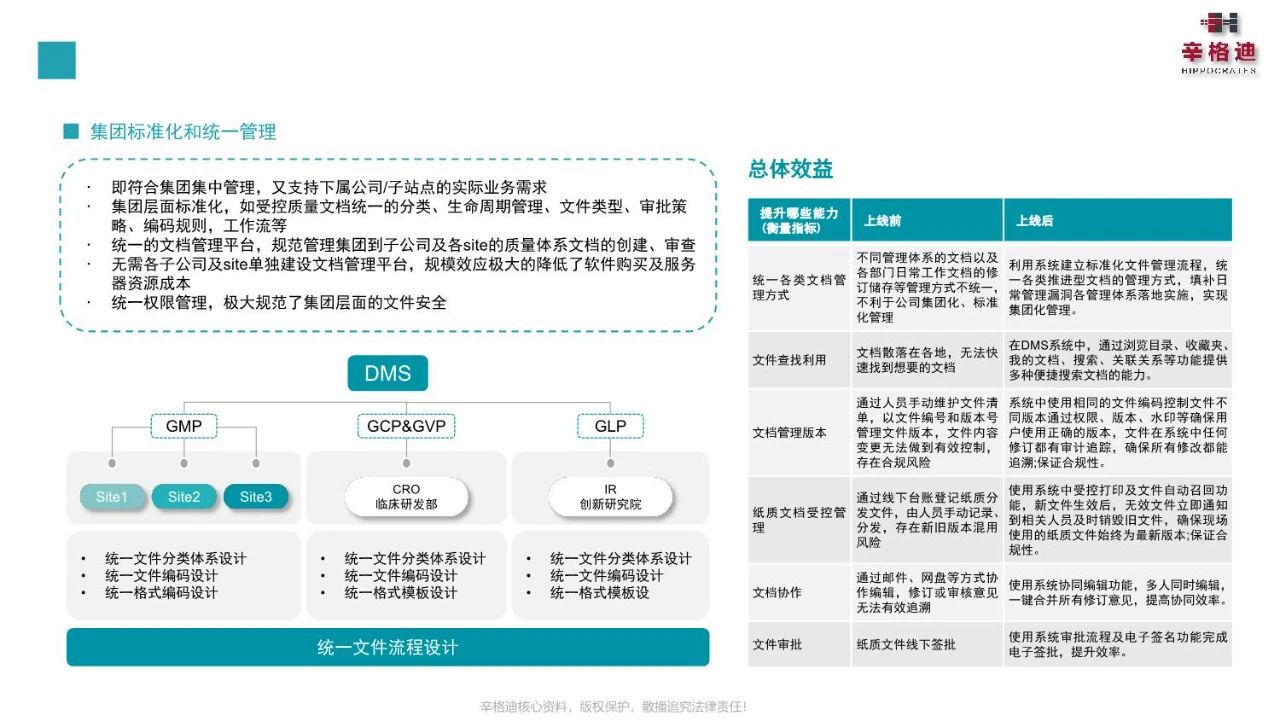
1. Support for Flexible Group Architectures
With the continuous expansion of enterprise scale and the diversified development of businesses, group-based operations have become a trend for enterprises in the life health sector. However, most existing document management systems on the market can barely meet the collaborative needs among multiple departments and subsidiaries under a group architecture.
The all-new DMS system launched by Singdi achieves comprehensive support for group enterprises through advanced technical architecture and functional design. It supports the management of multi-organization and parallel organizations, enabling connectivity between organizations at different levels to facilitate internal information sharing and collaboration. Whether it is collaboration between the headquarters and branches, or cross-business-line resource sharing, the system provides a seamlessly connected management experience. At the same time, the system can be customized and configured according to the group's organizational structure, supporting document management needs across multiple levels, departments, and subsidiaries. This greatly enhances the efficiency and transparency of document management for group enterprises.
2. Robust Permission Management
The new-generation DMS system stands out with its remarkable permission management capabilities. It has built a refined permission management system, and through careful design, it achieves strict permission control and data isolation, ensuring that documents can be securely shared and circulated among different organizations within the group.
Take a large multinational pharmaceutical group as an example: core members of the R&D department may have permission to edit and modify new drug R&D data, while employees from other departments can only view relevant summary information—effectively protecting the security of core data. Singdi's DMS realizes precise control over each level of permission through multi-level permission nesting, covering basic functions such as viewing and editing, as well as advanced functions like approval and permission assignment. Additionally, the system supports dynamic permission adjustment: when an employee's position changes or project tasks are modified, their document access permissions can be updated in real time, ensuring that permissions always match their responsibilities.
In setting document access permissions, full consideration is given to regulatory requirements for controlling different types of documents. For instance, documents involving key pharmaceutical production processes are only accessible and operable by specific personnel certified in compliance with regulations. This ensures that the enterprise's document management process meets regulatory standards from the source, reducing compliance risks.
3.Seamless Support for MAH Collaboration
The new-generation DMS system has also undergone comprehensive adaptation and upgrading for the Marketing Authorization Holder (MAH) business model. It has built a comprehensive and flexible document sharing and collaboration platform, allowing MAHs and their contracted parties to securely and conveniently share documents within the system. For example, an MAH can accurately share key pharmaceutical technical data with contracted manufacturers responsible for production, while setting corresponding permissions (for viewing, editing, and downloading) for personnel in different roles based on business needs—ensuring the confidentiality and integrity of documents during circulation. Through real-time collaboration functions, personnel from all parties can jointly edit documents, review them, and add comments online, significantly improving communication efficiency and shortening the document processing cycle.
Meanwhile, the system supports document version management and historical record tracking to ensure document accuracy and integrity. In terms of compliance assurance, the system strictly adheres to international authoritative regulatory standards such as GMP, ICH, and FDA. For various documents involved in MAH business, Singdi's new-generation DMS system conducts detailed compliance classification and control. During the pharmaceutical production process, key documents such as production process regulations and batch records are only accessible and operable by personnel who have obtained regulatory certification and corresponding qualifications—ensuring that the enterprise's document management process meets regulatory requirements from the source and effectively reducing compliance risks.
Additionally, the system fully records the operational history of documents (including creation, modification, and approval), providing clear and traceable evidence for inspections by regulatory authorities. It also features powerful version management capabilities. In MAH business, document versions are frequently updated (e.g., optimization of pharmaceutical formulas or improvement of production processes may lead to version changes). Singdi's new-generation DMS system can automatically identify document version changes and save all historical versions, allowing users to view and compare versions at any time. When it is necessary to trace the status of a document at a certain stage, users can retrieve the corresponding version with simple operations, avoiding work errors and compliance risks caused by version confusion.
The practical application effect is remarkable: after a well-known MAH enterprise introduced Singdi's new-generation DMS system, the efficiency of document transmission between the enterprise and its contracted manufacturing partners increased by 70%, and the average document approval cycle was shortened by 50%. Compliance issues caused by poor document management decreased by 85%, which significantly optimized the business collaboration process and improved the enterprise's operational efficiency and risk control capabilities.
Conclusion
The launch of Hippocrates 's new-generation DMS system will bring significant value to enterprises in the life health industry. Whether it is pharmaceutical companies, medical device firms, or biotechnology enterprises, all can leverage this system to achieve digital upgrading of document management and enhance their core competitiveness.
The COO of Hippocrates , who has experience in digital operations for many large multinational groups at home and abroad, stated: "For pharmaceutical and medical device enterprises, document management that meets compliance requirements is a fundamental aspect that enterprises must prioritize. The launch of Hippocrates 's new-generation DMS system not only addresses the pain points of group enterprises in document management but also provides them with an intelligent and integrated information management platform. We believe this system will become a powerful driver for the digital transformation of group enterprises."
Currently, Hippocrates 's new-generation DMS system has been officially launched and recognized by a considerable number of clients. In the future, Hippocrates will continue to uphold its original mission of "creating health value through technology," continuously optimize product functions, and contribute more to the digital transformation of the life health industry.
About Hippocrates
Hippocrates is a leading provider of digital solutions in China's life health sector. It is committed to helping life health enterprises (including those in pharmaceuticals, medical devices, and cosmetics) achieve digital transformation through innovative technologies and products. The company's business scope covers GMP quality management, digital factory construction, GVP pharmacovigilance, and GCP clinical research. It has served over 200 clients in total in China, established overseas branches, and formed cooperative relationships with a number of multinational enterprises.



