01 Project Background
Convalife (Shanghai) Health Technology Co., Ltd., referred to as Convalife Pharmaceuticals, is committed to drug R&D and production. Rooted in China and expanding globally, Convalife Pharmaceuticals focuses on the R&D of original innovative drugs and gradually enters the international pharmaceutical market. Hippocrates is dedicated to providing advanced, compliant, and efficient digital products and services for life science and healthcare enterprises, meeting international standards such as GMP and FDA, to support enterprises in their digital transformation.
The cooperation goal of both parties is to establish a high-standard compliance management platform, integrate internationally leading biopharmaceutical information technology, and jointly promote the R&D of drugs and new products. This cooperation will provide Convalife Pharmaceuticals with strong technical support and compliance guarantees, accelerating its development in the international pharmaceutical market. It will also promote Hippocrates' further development and innovation in the digital field of biopharmaceuticals. Through this cooperation, both parties will work together to enhance the international competitiveness of China's pharmaceutical industry and bring more high-quality medical solutions to global patients.
02 Project Kick-off Meeting
To improve the company's quality management and promote its development, Convalife (Shanghai) Health Technology Co., Ltd. recently held the kick-off meeting for the "Convalife eQMS Project". Participants included Convalife Pharmaceuticals' General Manager, relevant leaders, key directors from the Production Department, Technology Department, and Administration Department, as well as Hippocrates' Chief Operating Officer (COO), Regional Business Director, Director of Customer Success, and project team members. At the kick-off meeting, both parties conducted in-depth exchanges on quality compliance digitalization, including quality systems, document management, training management, pharmacovigilance, and project planning and prospects.
03 Leadership Speeches
Tan Ping, Chief Operating Officer (COO) of Hippocrates
At the kick-off meeting, Mr. Tan emphasized that Hippocrates has taken note of Convalife Pharmaceuticals' corporate strategy of being rooted in China and facing the global market. He pointed out that business compliance and process digitalization are the solid foundation for enterprises to move towards internationalization. Hippocrates' OwlTrust® Quality Compliance Digital Platform has won Convalife Pharmaceuticals' trust with its excellent performance and functions. This platform will provide comprehensive technical support and compliance guarantees for Convalife Pharmaceuticals, ensuring it maintains a leading position in the fierce market competition.
General Manager Shen of Convalife Pharmaceuticals
Mr. Shen stated that after in-depth and rigorous supplier evaluation and product selection, Convalife Pharmaceuticals ultimately decided to adopt Hippocrates' OwlTrust® Quality Compliance Digital Platform. The platform will bring standardized quality management and strict risk control to the company, while enhancing all employees' GMP awareness and the company's quality management level. Mr. Shen put forward clear expectations and requirements for the project team members, emphasizing that all departments must attach great importance to the project implementation to ensure the project progresses smoothly as planned and goes live soon, so as to achieve the company's long-term development goals.
04About the OwlTrust® Digital Platform
OwlTrust® is an integrated quality compliance digital platform developed by Hippocrates' experts in the GMP industry and digital field. It is built in strict accordance with standards and regulations such as GMP, cGMP, ICH, FDA 21 CFR Part 11, EU-GMP (ANNEX 11), and ISPE GAMP5. With the management concept and market positioning of "Quality Compliance Expert", it helps pharmaceutical enterprises improve their quality compliance level, meet industry supervision, accelerate the launch of more safe, effective, and high-quality products, and realize greater health value.
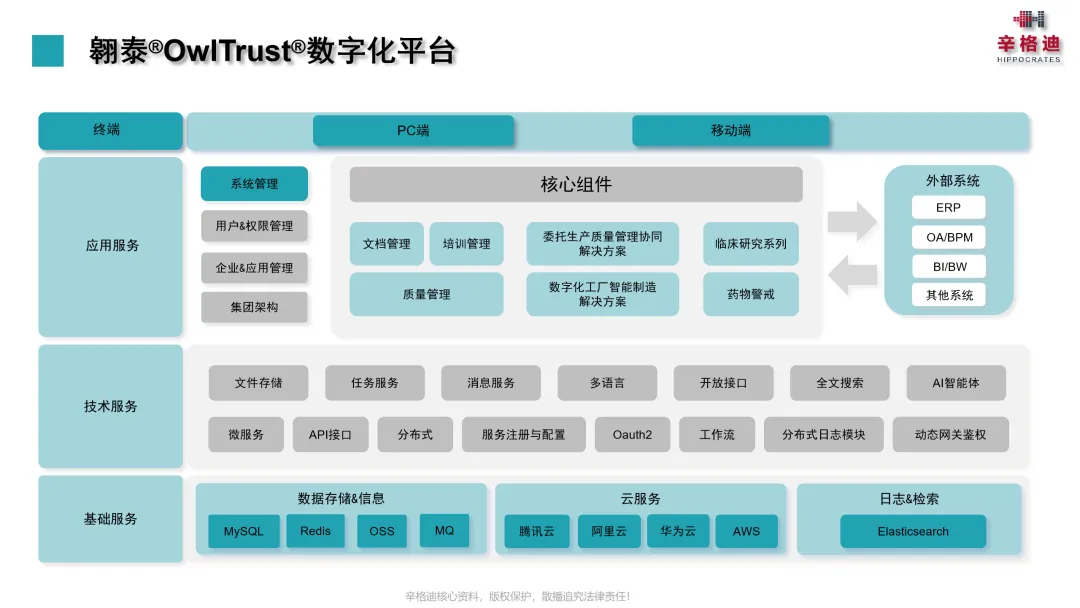
05Introduction of Both Parties
About Convalife Pharmaceuticals
Convalife (Shanghai) Health Technology Co., Ltd. is a clinical-stage innovative drug R&D enterprise rooted in China and facing the global market. The company focuses on the development of innovative drugs in the fields of oncology, virology, and aging-related diseases, and is committed to designing and developing original innovative drugs with "First-in-Class" or "Best-in-Class" potential. Convalife Pharmaceuticals' core team consists of professionals from top domestic and foreign pharmaceutical companies such as Novartis and Huahai Pharmaceutical, and maintains continuous cooperation with renowned research institutions such as the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
The company's product pipeline is extensive, covering various solid tumors such as breast cancer, lung cancer, cholangiocarcinoma, prostate cancer, pancreatic cancer, and gastric cancer, as well as hematological tumors.
In the past few years, Convalife Pharmaceuticals has successfully completed four rounds of financing and announced a strategic cooperation with Henlius (2696.HK), authorizing Henlius the exclusive commercialization rights in China and the exclusive negotiation and conditional licensing rights in agreed overseas countries and regions. The company's first independently developed product, Neratinib Maleate Tablets (trade name: Hannaijia®), was approved for marketing by the National Medical Products Administration (NMPA) on June 28, 2024, for adult patients with HER2-positive early breast cancer, and registration has started in six overseas countries. Currently, Convalife has entered a stage of rapid development, and several FIC and BIC product pipelines are in negotiations for cooperation with overseas companies.
About Hippocrates
"Hippocrates" is the transliteration of the "Father of Western Medicine" in ancient Greece. Hippocrates is a leading digital solution provider in China's life science and healthcare field, committed to helping life science and healthcare enterprises (pharmaceuticals, medical devices, cosmetics, etc.) achieve digital transformation through innovative technologies and products. The company's business covers GMP quality management, digital factory construction, GVP pharmacovigilance, GCP clinical research, etc. It has served more than 200 customers in China, established overseas branches, and formed cooperative relationships with multiple multinational enterprises.
The company's brands include Hippocrates Health and Relerson Consulting.



