

Cell and Gene Therapy Traceability Solution (CGTS)
For cell and gene therapy (CGT), paper-based records undoubtedly add to the already complex processes and regulatory challenges of CGT. Hippocrates's Cell and Gene Therapy Traceability Solution(CGTS) is designed to optimize the development and manufacturing of complex cell therapy processes. It monitors the manufacturing processes and supply chains of cell therapy facilities through a unified digital platform, helping enterprises ensure compliance, mitigate risks, and improve efficiency. The CGTS solution consists of three components: Chain of Identity/Chain of Custody (COI/COC), Manufacturing Management, and Quality Suite, as detailed in the figure below:
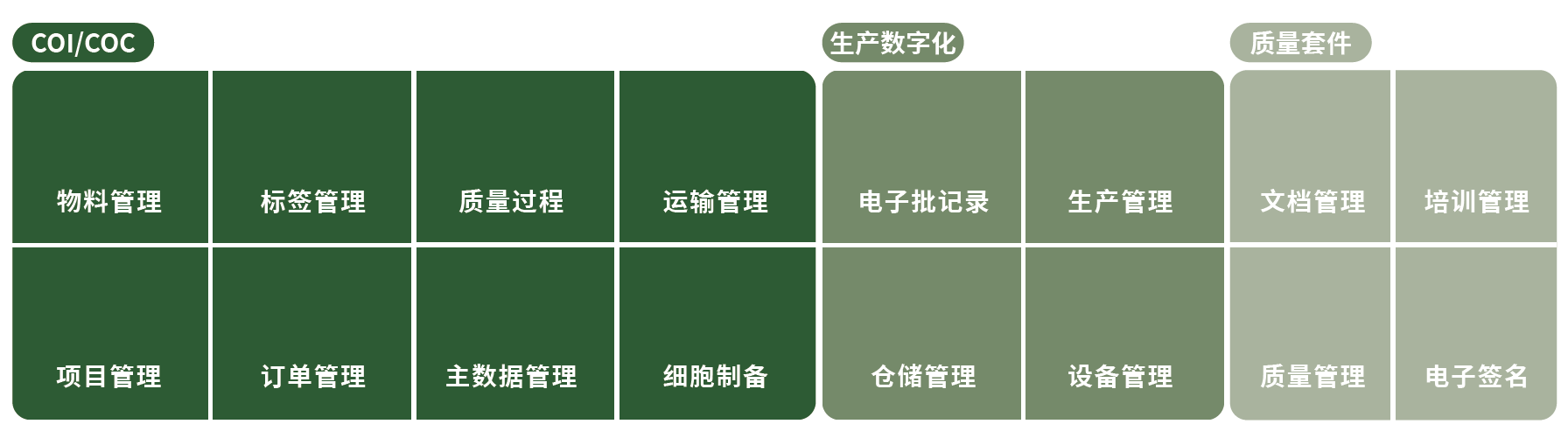
Based on international standards and best practices accumulated through years of experience in the Chinese domestic market, Hippocrates has launched a cell therapy traceability system that includes the Chain of Identity (COI) and Chain of Custody (COC). The implementation of COI and COC can effectively help you avoid the risk of misadministration of these specialized pharmaceuticals and prevent mix-ups and errors.
EBRs track every manufacturing step and improve production efficiency through automation. In addition, digital EBRs enhance compliance with Good Manufacturing Practice (GMP), improve product safety, and better meet regulatory requirements for cell and gene therapy products.
Our manufacturing management system ensures that personnel perform correct operations and maintain accurate records, while reducing the time spent on record-filling and record management to improve production efficiency. Moreover, through the system’s recording and management of production records, equipment information, and environmental data, automation of the production process is achieved.
The barcode and Internet of Things (IoT)-based warehouse management system supports quality status management, batch/lot number management, and inbound/outbound management of pharmaceuticals and raw materials. Combined with electronic supervision code management, it effectively prevents mix-ups and errors in logistics, warehousing, and pharmaceuticals.
Dynamically connected instruments and equipment enable real-time acquisition of equipment status and sensor readings (e.g., usage status and conditions such as temperature, pressure, and humidity). This avoids quality issues caused by abnormal equipment conditions and allows for preventive maintenance of equipment failures. Meanwhile, this data also provides rich support for process characterization and effective root cause analysis, helping to reduce process development cycle time and costs.
Quality management includes document management, training management, as well as deviation management, Corrective and Preventive Actions (CAPA) management, and release management. Through digital methods, it ensures the correct implementation of Standard Operating Procedures (SOPs) and provides timely training for relevant employees.
The Quality Suite helps enterprises achieve record compliance and data integrity, providing strong support for enterprises undergoing data integrity inspections by regulatory authorities such as the National Medical Products Administration (NMPA), U.S. Food and Drug Administration (FDA), and European Medicines Agency (EMA).
Additionally, we provide virtual data rooms (VDRs) and electronic signatures:
The administration method of cell therapy products differs from that of traditional pharmaceuticals. For example, cell therapy requires cell extraction, isolation, modification, cultivation, and subsequent infusion, involving complex processes and higher requirements for quality systems.
Cell therapy products have extremely high environmental requirements. As living cell products, their production is inherently variable—especially for autologous cell therapy products, where each batch is unique to an individual patient. Maintaining records manually or on paper is labor-intensive, error-prone, and incurs high storage costs. For subsequent Quality Assurance (QA) and Quality Control (QC) processes, paper-based records significantly increase the complexity, cost, and scalability challenges of the production process.
Hippocrates’s digital Cell and Gene Therapy Traceability Solution helps enterprises better meet GMP regulatory requirements, optimize production processes, and comprehensively improve production management capabilities. The specific benefits are as follows:
Hippocrates’s cell therapy solution offers functional modules suitable for different stages of enterprise development. Enterprises can select a solution that aligns with their development stage through modular configuration to initiate digitalization early, ensure pharmaceutical compliance with regulatory requirements, and accelerate product market launch.
Hippocrates’s CGTS is built on the Aotai Platform. Aotai is a digital platform jointly developed by Xingdi’s industry experts and IT specialists, with "Quality and Compliance Expert" as its management philosophy and market positioning. It helps enterprises improve quality and compliance standards, meet industry regulations, enhance management capabilities, and accelerate the market launch of more safe, effective, and high-quality products.
(2) Application Architecture
Applications on the Aotai Platform can be customized to meet an enterprise’s quality system requirements. The built-in configuration tools already include multiple functions of quality solutions, eliminating the need for customization or programming to implement certain special processes. Trained personnel can quickly customize forms, decision trees, and dashboards to meet specific business needs.
(3) Business Architecture
Hippocrates’s cell therapy solution realizes business planning and digital functions through four phases, greatly helping enterprises free themselves from cumbersome manufacturing and supply chain processes, allowing them to focus solely on improving the efficacy and safety of cell and gene therapy drugs.
(4) Technical Architecture
Quality and compliance processes are built on commonly used master data, which only needs to be set up once and can be used across different modules of the entire system. New processes automatically reference these data dictionaries, ensuring that enterprises continue to use a "common language" as their quality system evolves.
Features of the technical architecture include:
The superior design of the Aotai Platform ensures that users can quickly access large volumes of records and data, thereby improving work efficiency.